Dr. Anne Bang is an experienced cell biologist and stem cell expert who leads efforts at the Prebys Center to develop patient cell specific and human induced pluripotent stem cell (hiPSC)-based disease models for drug screening and target identification. Dr. Bang has over 20 years of experience in the fields of developmental and stem cell biology. She obtained a BS degree from Stanford University, a PhD in Biological Sciences from the University of California, San Diego, and did postdoctoral training in the Neurobiology Laboratory at the Salk Institute for Biological Sciences where her studies focused on nervous system development.
Anne’s experience in stem cell biology began in 2005 when she joined ViaCyte, Inc. where she served as Director of Stem Cell Research and managed an interdisciplinary group working to develop human embryonic stem cells as a replenishable source of pancreatic cells for the treatment of diabetes. Her efforts focused on optimization of the differentiation process, and then on advancing the cell therapy product into development, scaled manufacturing, product characterization, and safety assessment. Anne is a co-inventor on multiple ViaCyte patents, and her team’s contributions played a key role in securing a $20 MM California Institute of Regenerative Medicine (CIRM) Award.
In June of 2010, Sanford Burnham Prebys recruited Anne as Director of Cell Biology to lead efforts in stem cell-based disease modeling at the Conrad Prebys Center for Chemical Genomics. Her role includes leading internal research projects, as well as external collaborations with academic and industry partners. Anne’s research program is primarily focused on neurological and neuromuscular disease, with the aim of designing human cell-based models and assays that recapitulate disease phenotypes, yet have the throughput and reproducibility required for drug discovery. Towards this goal her group has worked to develop a suite of foundational high throughput assays to monitor neuronal morphology, mitochondrial function, and electrophysiology, using high content screening, and multi-electrode array formats. They have conducted high-throughput drug screens on muscular dystrophy patient cells, hiPSC-derived cardiomyocytes, and hiPSC-derived neurons, including from Alzheimer’s patient specific hiPSC. Anne is a principal investigator for the National Institute of Mental Health (NIMH) National Cooperative Reprogrammed Cell Research Groups consortium and has also received research support from rare disease foundations and pharma sponsored collaborations. She also serves on advisory boards for multiple biotechnology companies.
Related Disease
Aging-Related Diseases, Alzheimer’s Disease, Amyotrophic Lateral Sclerosis (Lou Gehrig’s Disease), Congenital Disorders of Glycosylation, Glycosylation-Related Disorders, Multiple Sclerosis, Muscular Dystrophy, Neurodegenerative and Neuromuscular Diseases
Phenomena or Processes
Cell Biology, Development and Differentiation, Development of Neuronal Circuits, Embryonic/Pluripotent Stem Cells, Neurobiology, Neurodegeneration, Neurogenesis, Neuron-Glia Interactions in Myelin, Neurotransmitters, Synapse Formation and Maturation, Synapse Function, Synaptic Transmission
Research Models
Human Adult/Somatic Stem Cells, Human Cell Lines, Human Embryonic Stem Cells
Techniques and Technologies
Cellular and Molecular Imaging, Drug Discovery, Electrophysiology, Fluorescence Microscopy, High Content Imaging, In vivo Modeling, Microarrays, Molecular Genetics
Dr. Anne Bang is an experienced cell biologist and stem cell expert who leads efforts at the Prebys Center to develop patient cell specific and human induced pluripotent stem cell (hiPSC)-based disease models for drug screening and target identification. Her research program is primarily focused on neurological and neuromuscular disease, with the aim of designing human cell based models and assays that reflect higher order cellular functions and recapitulate disease phenotypes, yet have the throughput and reproducibility required for drug discovery. Towards this goal her group has worked to develop a suite of foundational high throughput assays to monitor neuronal morphology, mitochondrial function, and electrophysiology, using high content screening, and multi-electrode array formats. They have conducted high-throughput drug screens on muscular dystrophy patient cells, hiPSC-derived cardiomyocytes, and hiPSC-derived neurons, including from Alzheimer’s patient specific hiPSC. Anne is a principal investigator for the National Institute of Mental Health (NIMH) National Cooperative Reprogrammed Cell Research Groups consortium, and also receives research support from rare disease foundations and pharma sponsored collaborations.
Anne Bang’s Research Report
Publications
- High content screen for compounds that modulate neurite outgrowth and retraction of human induced pluripotent stem cell derived neurons. Sherman, S.P. and A.G. Bang. Dis Model Mech. 2018 Feb 2;11(2):dmm031906.
- AAV-Mediated Mini-Agrin Delivery is Unable to Rescue Disease Phenotype in a Mouse Model of Limb Girdle Muscular Dystrophy Type 2I. Vannoy, C.H., H. Zhou, C. Qiao, X. Xiao, A.G. Bang*, Qi L. Lu* Am J Pathol. 2017 Feb;187(2):431-440. (*corresponding authors).
- Neuronal medium supporting physiological synaptic activity and fundamental functions of human neurons in vitro. Bardy C., M. Van Den Hurk, C. March and, T. Eames, R. Hernandez, M. Kellogg, M. Gorris, B. Galet, V. Palomares, J. Brown, A.G. Bang, J. Mertens, L. Boehnke, L. Boyer, S. Simon, F. H. Gage. Proc Natl Acad Sci U S A. 2015 May 19;112(20):E2725-34.
- A Scalable System for Production of Functional Pancreatic Progenitors from Human Embryonic Stem Cells. Schulz T., H.Y. Young, A.D. Agulnick, J. Babin, E.E. Baetge, A.G. Bang, A. Bhoumik, et al. PLoS One. 2012;7(5):e37004.
- Novel surface markers for the isolation and transplantation of pancreatic cells derived from human embryonic stem cells. Kelly O.G., M.Y. Chan, L.A. Martinson, K. Kadoya, T. Ostertag, K. Ross, M. Richardson, M.K. Carpenter, K.A. D’Amour, E. Kroon, M. Moorman, E.E. Baetge, and A.G. Bang. Nat Biotechnol. 2011 Jul 31;29(8):750-6.
- Characteristics and characterization of human pluripotential stem cells. Bang A.G. and M.K. Carpenter. In Essentials of Stem Cell Biology, 2009 Nov 30;(38)339-343
- Deconstructing pluripotency. Bang A.G. and M.K. Carpenter. Science 2008 Apr 4;320(5872):58-9.
- Pancreatic endoderm derived from human embryonic stem cells generates glucose responsive insulin-secreting cells in vivo. Kroon E., L. A. Martinson, K. Kadoya, A.G. Bang, O.G. Kelly, S. Eliazer, H. Young, M. Richardson, N.G. Smart, J. Cunningham, A.D. Agulnick, D’Amour KA, M.K. Carpenter & E.E. Baetge. Nat Biotechnol. 2008 Apr;26(4):443-52.
- Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. D’Amour K.A,. A.G. Bang, S. Eliazer, O.G. Kelly, A.D. Agulnick, N.G. Smart, M.A. Moorman, E. Kroon, M.K. Carpenter & E.E. Baetge. Nat Biotechnol. 2006 Nov;24(11):1392-401
Patents
- Jackson, M., and Bang, A.G. 2013. Theranostics platform and methods of use, Patent Application No. PCT/US13/26448, Sanford Burnham Medical Research Institute, La Jolla, CA.
- Kelly, O.G., and Bang, A.G. 2012. Methods for purifying endoderm and pancreatic endoderm cells from human embryonic stem cells, United States Patent 8,338,170, ViaCyte, Inc., San Diego, CA.
- D’Amour K., Bang A., Baetge, E. 2012. Endocrine precursor cells, pancreatic hormone-expressing cells and methods of production. United States Patent 8,129,182, ViaCyte, Inc., San Diego, CA.
- Green, C., Yu X., Bang A., Brandon, E., Kelly, O., Agulnick, A., Baetge, E., D’Amour K., Schulz, T., Robins, A. 2011. Stem cell aggregate suspension compositions and methods of differentiation thereof. United States Patent 8,008,075, ViaCyte, Inc., San Diego, CA.
 Oct 11, 2024
Oct 11, 2024Anne Bang joins $12.7M research project on the genetic basis of autism and schizophrenia
Oct 11, 2024Sanford Burnham Prebys scientist and an international team are funded by the California Institute for Regenerative Medicine.
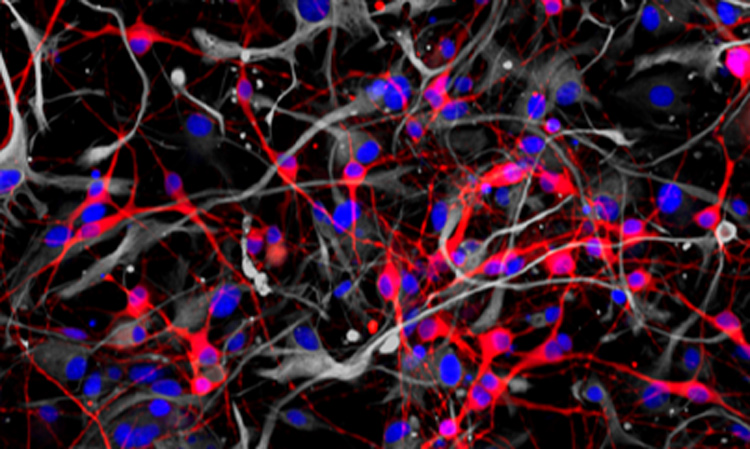 Aug 18, 2022
Aug 18, 2022Using stem cells to study the biochemistry of learning
Aug 18, 2022A method for studying human neurons could help researchers develop approaches for treating Alzheimer’s, schizophrenia and other neurological diseases.
 Dec 8, 2020
Dec 8, 2020What scientists are learning about COVID-19 and the brain
Dec 8, 2020We caught up with cell biologist Anne Bang, who recently teamed up with her husband to study how SARS-CoV-2 affects…
 Feb 7, 2019
Feb 7, 2019Drug screen conducted at Sanford Burnham Prebys identifies new therapeutic avenues for Alzheimer’s disease
Feb 7, 2019A screen of more than 1,600 Food and Drug Administration (FDA)–approved drugs performed at SBP’s Conrad Prebys Center for Chemical
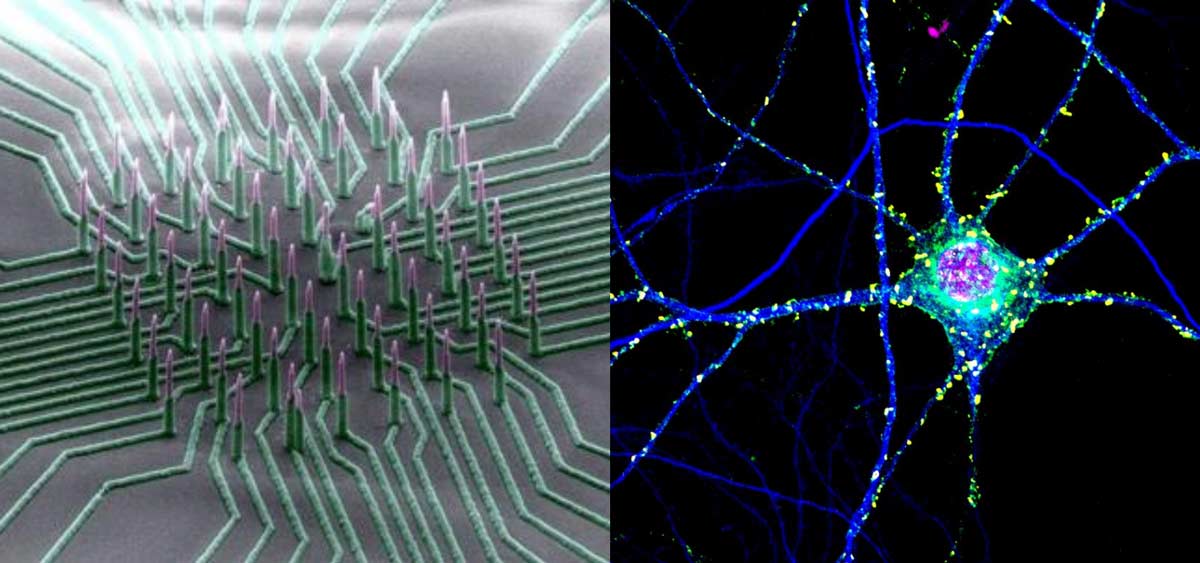 Apr 12, 2017
Apr 12, 2017Nanowire arrays allow electrical recording of neuronal networks
Apr 12, 2017To examine a neuron’s health, activity and response to drugs, scientists record its electrical activity. Current methods of recording are
 Aug 31, 2016
Aug 31, 2016Consortium awarded $15 million to unravel bipolar disorder and schizophrenia
Aug 31, 2016Sanford Burnham Prebys Medical Discovery Institute (SBP), the Johns Hopkins University School of Medicine, the Salk Institute for Biological Studies,…
