Pamela Itkin-Ansari earned her PhD in Biomedical Sciences from the University of California, San Diego, in 1999. She received postdoctoral training focused on diabetes at that same organization. In 2003, Dr. Itkin-Ansari was appointed Assistant Professor in the Department of Pediatrics, UC San Diego. She moved her laboratory to Sanford Burnham Prebys in 2005.
Funding Awards and Collaborative Grants
California Institute for Regenerative Medicine (CIRM)
Falk Foundation
The Hartwell Foundation
The Hirshberg Foundation
Juvenile Diabetes Research Foundation (JDRF)
National Institutes of Health
Select Honors and Recognition
2013-2014: Outstanding Faculty Mentor Award, UCSD
2011-2014: Hartwell Foundation Biomedical Research Award
2011: Invited Speaker, TEDx
2008-2013: Board of Directors, JDRF San Diego
2008: Health Hero Leadership Award, Combined Health Agencies of San Diego
Other Affiliations
2012-current: Islet Society
2010-current: ASGCT
2008-current: Board of Directors, JDRF San Diego chapter
2008-2013: JDRF board of directors, San Diego
2007-current: American Association for Cancer Research
2007-current: American Diabetes Association
2007-current: American Pediatric Society/Society for Pediatric Research
2006-current: AAAS
Related Disease
Cancer, Diabetes – General, Gastric Cancer, Monogenic Diabetes, Pancreatic Cancer, Type 1 Diabetes, Type 2 Diabetes
Dr. Itkin-Ansari’s research is directed toward understanding diseases of the human pancreas.
Pamela Itkin-Ansari’s Research Report
Diabetes
Areas of focus are: 1) developing a cell-based therapy for diabetes that does not require immunosuppression, and 2) identifying proteins required for proper insulin production and processing.
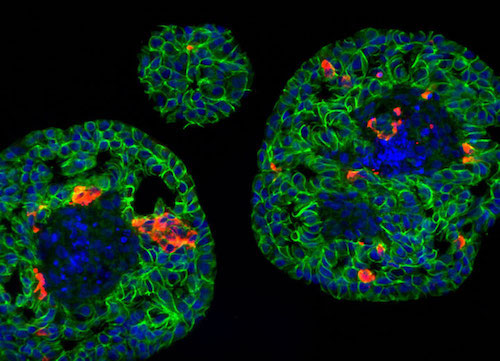
Islet clusters in the developing pancreas
Pancreatic Cancer
The lab determined how dysregulation of specific transcription factors triggers pathogenic cell cycle entry in normal pancreatic cells. This master signaling pathway controlling pancreatic cancer cell growth is yielding potential targets for drug discovery.
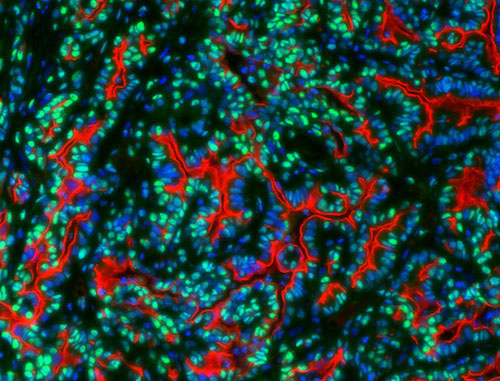
Id3 (green) in human pancreatic cancer
 Sep 24, 2025
Sep 24, 2025How a Holocaust survivor transformed blood sugar testing
Sep 24, 2025New podcast discusses how a scientist saved from a World War II concentration camp revolutionized diabetes care.
 Aug 19, 2025
Aug 19, 2025Seeing how sugar reshapes our blood
Aug 19, 2025Scientists and podcasters tackle how a scientist in Iran overcame great odds and forever changed diabetes diagnoses.
 May 27, 2025
May 27, 2025Scientists and podcasters
May 27, 2025Sanford Burnham Prebys scientists bring dramatic stories of scientific achievement to life.
 May 22, 2024
May 22, 2024Pancreatic cancer symposium celebrates 10th anniversary in San Diego
May 22, 2024The 2024 PancWest Symposium brought more than 120 scientists to the Sanford Burnham Prebys campus in San Diego to discuss…
 Mar 28, 2023
Mar 28, 2023Behind the scenes at Sanford Burnham Prebys’ Cancer Center
Mar 28, 2023Cancer Center open house connects San Diego community with scientists working toward cancer cures
 Oct 26, 2022
Oct 26, 2022Research unlocks the circuitry of diabetes
Oct 26, 2022Research led by Pamela-Itkin-Ansari, PhD, and Randal Kaufman PhD, has mapped out a network of biochemical interactions that help special…
