Dr. Wechsler-Reya’s research focuses on the signals that control growth and differentiation in the cerebellum, and how these signals are dysregulated in the brain tumor medulloblastoma. As a postdoc, he demonstrated that Sonic hedgehog (Shh) is a critical mitogen for neuronal precursors in the cerebellum, and that mutations in the Shh pathway predispose to medulloblastoma by activating a mitogenic pathway that normally functions only in early development.
Now in his own lab, he continues to study the relationship between brain development and brain tumor formation. His lab’s contributions include identifying N-myc as a key target of the Shh pathway in neuronal precursors and in tumor cells; discovering a novel population of neural stem cells in the neonatal cerebellum; demonstrating that both neuronal precursors and stem cells can serve as cells of origin for MB; and identifying a population of cancer stem cells that is critical for propagation of Shh-associated tumors.
More recently, Dr. Wechsler-Reya and his group have begun developing new models of medulloblastoma and are using them to test novel therapeutic approaches. His work has garnered several awards, including a Kimmel Scholar Award, an Award for Excellence in Pediatrics Research from the Society for Neuro-Oncology and a Leadership Award from the California Institute for Regenerative Medicine (CIRM).
Education
2001-2010: Associate Professor of Pharmacology and Cancer Biology, Duke University Medical Center
1997-2001: Postdoctoral Fellow, Stanford University, Neural Development
1995-1996: Postdoctoral Fellow, Wistar Institute, Molecular Oncology
1995: PhD, University of Pennsylvania, Immunology
1986: B.A., Harvard College, Psychology & Biology
Funding Awards and Collaborative Grants
Leadership Award from the California Institute for Regenerative Medicine (CIRM)
Other Affiliations
2012: 19th International Brain Tumor Research and Therapy Conference, University of Toronto, Niagara Falls, ON
2012: “Developmental tumors of the nervous system,” held in Barcelona on July 2012, as part of the 8th Forum of European Neuroscience Societies.
Honors and Recognition
2007: W.K. Joklik Award for Excellence in Basic Cancer Research
2007: DukeMed Scholar
2006: Award for Excellence in Pediatrics Research, Society for Neuro-Oncology
2003: Kimmel Scholar Award, Sidney Kimmel Foundation for Cancer Research
2003: Brain Tumor Society Research Award
2002: Children’s Brain Tumor Foundation Research Award
2000-2001: Postdoctoral Fellowship, American Cancer Society (California)
1995-1997: Postdoctoral Fellowship, Medical Research Council of Canada
1988: Award for Excellence in Scientific Writing, American Diabetes Association
1984-1985: John Harvard Scholarship for Academic Achievement of Highest Distinction
Related Disease
Brain Cancer, Childhood Diseases
The Wechsler-Reya Lab studies the signals that control cell growth and differentiation in the nervous system and how these signals are dysregulated in brain tumors. We focus on medulloblastoma, the most common malignant brain tumor in children, and use models to understand the disease and to develop novel approaches to therapy. Our current areas of interest include:
- Discovering oncogenic drivers and creating new models
- Elucidating the molecular mechanisms of metastasis
- Identifying new therapeutics and approaches to drug delivery
- Harnessing the immune system to target tumors
We also work closely with physicians at Rady Children’s Hospital and elsewhere to translate our findings into trials that can benefit patients. Our goal is to develop safer and more effective therapies for children with brain tumors.
Robert Wechsler-Reya’s Research Report
Discovering Oncogenic Drivers and Creating Models
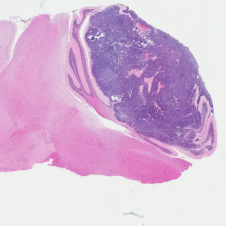
H&E stained Myc-Gfi1 tumor
Aggressive therapies – including surgery, radiation and high-dose chemotherapy – have improved outcomes for medulloblastoma patients, but many patients still die of their disease, and survivors suffer severe long-term side effects from therapy. To develop safer and more effective treatments, we need to understand the genes and pathways that are important for tumorigenesis. But for many forms of medulloblastoma, the oncogenic drivers are still unknown. A major focus of our research is to identify these drivers and use them to create robust animal models of the disease. Sequencing studies have identified genes that are altered in human medulloblastoma, and we are using functional assays to determine which of these genes can promote tumor growth in vivo. We have also established a large bank of patient-derived xenograft models that we use to perturb candidate genes and test their roles in tumorigenesis. In addition to identifying new drivers of medulloblastoma, these studies generate models that can be used to test novel approaches to therapy.
Elucidating the Molecular Mechanisms of Metastasis
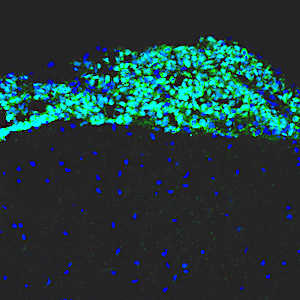
Leptomenigeal metastasis section
Most medulloblastoma research has focused on the primary tumors growing in the cerebellum. However, most medulloblastoma patients do not die from the primary disease, but from leptomeningeal metastasis: the dissemination of tumor cells from the cerebellum into the brain and spinal cord. Metastatic lesions cannot be surgically removed, and there are no effective therapies to eliminate them or stop them from growing. To understand the molecular basis of metastasis, we are using animal models to study the differences between primary and metastatic tumors, and screening for genes that can promote or inhibit metastatic dissemination. We are also integrating our results with molecular data from medulloblastoma patients, to identify genes that are critical for metastasis. Understanding the function of these genes will allow us to design novel strategies for early detection, prevention, and treatment of metastasis in patients with medulloblastoma and other types of brain tumors.
Identifying new Therapeutics and Approaches to Drug Delivery

Tumor-homing peptides in MP tumor
Genomic analyses have revealed that medulloblastoma is an extremely heterogeneous disease, with at least 4 distinct subtypes that differ in terms of mutations, gene expression, epigenetic changes, and patient survival. Despite this heterogeneity, most medulloblastoma patients currently receive the same therapy. A major goal of our research is to discover new therapeutic strategies that are tailored to specific medulloblastoma subgroups. To this end, we have assembled a large panel of patient-derived xenografts and are using them for high-throughput drug screening. Working with experts in genomics and computational biology, we are using statistical and mathematical tools to understand the relationship between molecular alterations and drug responses. These studies not only highlight new targeted therapies for medulloblastoma, but also provide insight into drug response biomarkers and help prioritize agents for clinical trials.
In addition to identifying therapeutics, we are also exploring novel approaches to drug delivery. A major obstacle to treating brain tumors is that the majority of small molecule drugs are not able to cross the blood brain barrier, and those that do are often pumped out by multi-drug transporters. To solve this problem, we are collaborating with bioengineers with expertise in nanotechnology. By encapsulating drugs in nanoparticles and delivering them directly to the central nervous system, we hope to increase the concentration of drugs in brain tumors and reduce the concentrations in other tissues, thereby mitigating systemic side effects. We have already identified several drugs that are effective at killing medulloblastoma cells in vitro; if we can develop strategies for effective delivery of these drugs to tumors, we can markedly improve outcomes for medulloblastoma patients.
Harnessing the Immune System to Target Tumors
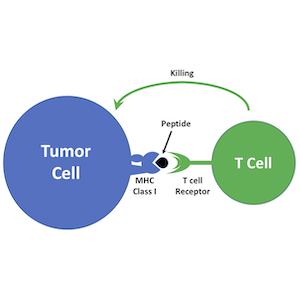
Immunotherapy is emerging as a powerful approach to treating cancer. Antagonists of immune checkpoint regulators, T lymphocytes engineered to recognize tumor antigens, and vaccines that amplify tumor-specific lymphocytes are being tested against a variety of human malignancies. Although some remarkable successes have been reported, only a subset of patients respond to these therapies, and the mechanisms that underlie resistance are poorly understood. Pediatric brain tumors, in particular, have not yet benefited from immunological targeting. We are studying the mechanisms brain tumors use to evade the immune system and suppress immune responses, and developing therapeutic strategies to overcome these mechanisms. We are also using genomic and proteomic approaches to identify antigens that might represent novel targets for vaccines, CAR T cells and natural killer cells. Finally, we are “humanizing” our PDX models so we can explore interactions between the immune system and patient-derived tumor cells. By increasing the immunogenicity of tumor cells and enhancing anti-tumor immune responses, we hope to bring the benefits of immunotherapy to medulloblastoma patients.
 Jul 6, 2022
Jul 6, 2022Heating up cold brain tumors: An emerging approach to medulloblastoma
Jul 6, 2022Immunotherapy has revolutionized cancer treatment, but it doesn’t work on many childhood brain tumors. Researchers from Sanford Burnham Prebys are…
 Apr 7, 2021
Apr 7, 2021Conrad Prebys Foundation provides $3 million for pediatric brain cancer research
Apr 7, 2021Conrad Prebys was an extraordinary man and a passionate philanthropist. Today, his generosity extends beyond his life through the Conrad…
 Dec 14, 2020
Dec 14, 2020Our top 10 discoveries of 2020
Dec 14, 2020This year required dedication, patience and perseverance as we all adjusted to a new normal—and we’re proud that our scientists
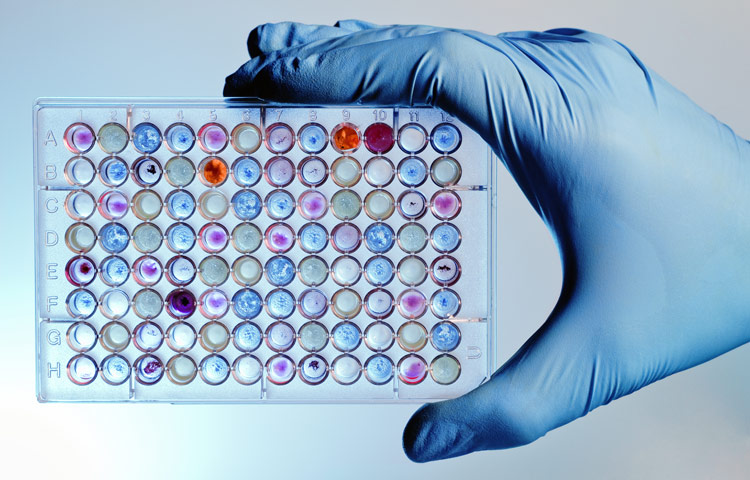 Nov 9, 2020
Nov 9, 2020Personalized drug screens could guide treatment for children with brain cancer
Nov 9, 2020A clinical trial that evaluates the approach for medulloblastoma, the most common malignant pediatric brain tumor, is planned Scientists at…
 Jul 9, 2020
Jul 9, 2020Sanford Burnham Prebys researchers awarded 2020 Padres Pedal the Cause grants
Jul 9, 2020We are pleased to announce that Padres Pedal the Cause (PPTC) has awarded three collaborative research grants to Sanford Burnham…
 Jan 13, 2020
Jan 13, 2020How to help children survive—and thrive—after a brain cancer diagnosis
Jan 13, 2020Lynne Selinka knew in her heart that something was seriously wrong with her 10-year-old son, Travis. For months he had…
